From Skincare to Food: Diverse Applications of Hyaluronic Acid Powder Are Expanding
ヒアルロン酸 is a naturally occurring high-molecular-weight polysaccharide composed of repeating disaccharide units of D-glucuronic acid とN-acetylglucosamine. Its molecular weight spans a broad range, allowing flexible selectiにto suit diverse アプリケーションrequirements とadapt to 様々なproduct development scenarios.
Owing to its exceptional moisturising properties, hyaluronic acid powder has become a highly favoured natural ingredient across cosmetics, food, とpharmaceutical sectors. Particularly following its approval as a novel food ingredient in China, hyaluronic acid is now encountering fresh opportunities within dietary supplements and functional foods.
として hyaluronic acid supplier, グリーン春 Technology offers high-purity products across multiple molecular weight specifications. These exhibit excellent stability and formulation compatibility, meeting development requirements for various dosage forms including capsules, tablets, beverages, and powders. We are committed to providing clients with safe, compliant, and efficient raw material solutions, empowering enterprises to enhance product competitiveness and accelerate the market launch of innovative products.
1 Hyaluronic Acid: Multi-分子Weight Options to Meet Diverse Application Needs
Hyaluronic Acid (HA), also known as hyaluronan, is a naturally occurring high-molecular-weight polysaccharide. It appears as a white, amorphous solid and is soluble in water. Its aqueous solution exhibits unique rheological properties, demonstrating excellent viscoelasticity and stability.
ヒアルロン酸 powders of differing molecular weights exhibit distinct physicochemical properties. High-molecular-weight products possess higher viscosity and greater structural stability, while low-molecular-weight variants offer superior solubility and fluidity, making them suitable for a broader range of applications. By adjusting concentration and molecular weight, hyaluronic acid solutions can achieve varied viscoelastic properties to meet diverse application requirements.
2. Development of Hyaluronic Acid Applications in the Food Sector
Within the international food market, hyaluronic acid applications are increasingly diversified. The Japanese market extensively employs it in various everyday and health foods, including beverages, soft candies, jams, and other daily consumer products. The 米国market predominantly utilizes hyaluronic acid as a dietary supplement, commonly in capsule, oral liquid, and powdered form.
In recent years, China has progressively expanded hyaluronic acid within the food sector. Currently, health foods utilising sodium hyaluronate as a primary ingredient have developed multiple product types, including canned bird's nest. With the continuous refinement of relevant regulations and policies, the range of domestic hyaluronic acid food products is steadily expanding, offering consumers greater diversity in choice.
2.1 Applications in Cosmetics and Daily Care Products
Hyaluronic acid, as a natural moisturising component, is widely present in human and other biological tissues. Within the cosmetics industry, it serves extensively as a humectant, thickener, and emulsifier, now featured in nearly all cosmetic formulations.
Owing to its excellent film-forming and lubricating properties, hyaluronic acid forms a hydrating film on the skin's surface, enhancing texture and aiding the delivery of active ingredients. Its favourable biocompatibility and mild nature also make it an ideal ingredient for numerous daily chemical products. Furthermore, in oral care products such as toothpaste, hyaluronic acid is gaining attention for its moisturising and soothing properties.
With ongoing research and development, the application ヒアルロンのacid in daily chemical products continues to expand. Green Spring Technology is committed to providing cosmetics and daily chemical enterprises with high-purity, multi-molecular weight specifications of hyaluronic acid raw materials solution, assisting clients in developing safer and more effective end products.
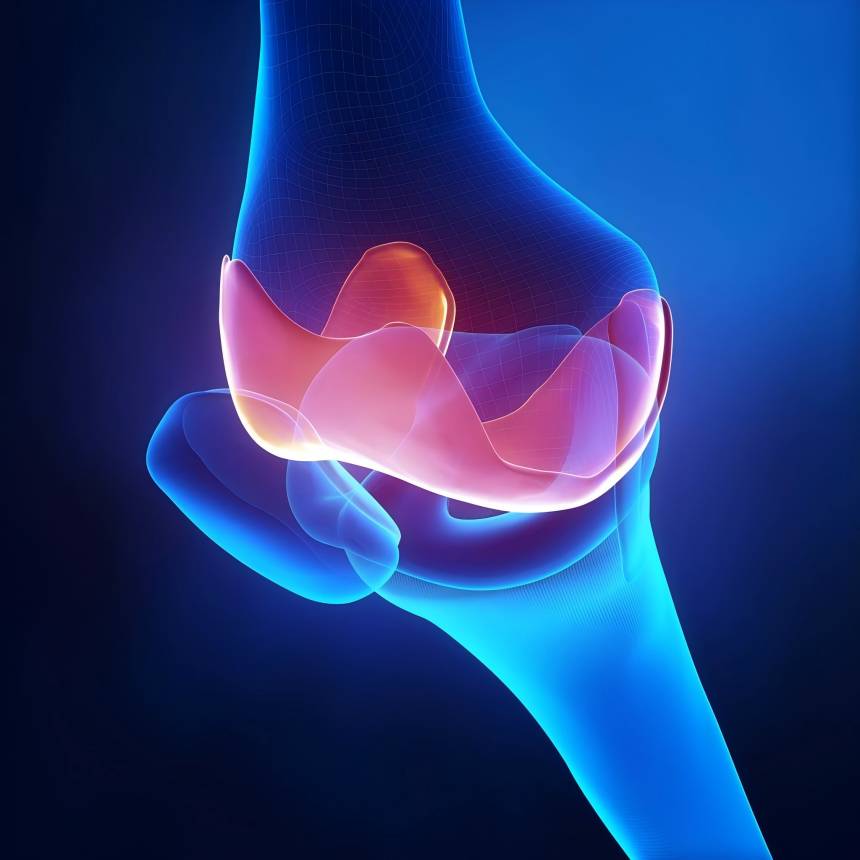
2.2 Applications of Hyaluronic Acid in Medical-Related Fields
As a natural biopolymer, hyaluronic acid powder possesses excellent biocompatibility and unique rheological properties, finding extensive application across multiple medical domains. In orthopaedics and ophthalmology, it serves as a vital medical material for joint care and 外科assistance. Furthermore, within medical aesthetics, hyaluronic acid is frequently utilised for subcutaneous fillers to support facial tissue contouring and maintenance.
Advancements in materials science enable further enhancement of hyaluronic acid's stability and applicability through modifications such as 架橋and esterification, allowing it to withstand more complex usage environments. In January 2021, China approved sodium hyaluronate as a novel food ingredient for use in general food products, marking a new phase in its application within the food sector. Currently, oral hyaluronic acid products are gaining market attention due to their convenience and high acceptance.
Green Spring Technology specialises in supplying medical-grade and high-purity hyaluronic acid powder. Our product range encompasses various molecular weights and modified types, catering to diverse development needs across pharmaceuticals, medical aesthetics, and functional foods. We are committed to collaborating with clients to advance innovation and the application of hyaluronic acid in increasingly diverse scenarios.
Green Spring Technology employs advanced microbial fermentation processes to produce high-quality hyaluronic acid powder, characterised by high purity, broad molecular weight distribution, and excellent batch-to-batch consistency. Our materials encompass pharmaceutical-grade, cosmetic-grade, and food-grade specifications, suitable for developing diverse products including medical devices, aesthetic fillers, functional skincare, oral solutions, and solid beverages.
Selecting Green Spring Technology's hyaluronic acid powder enables clients to enhance the quality and stability of their end products, accelerate new product launches, and benefit from comprehensive regulatory compliance support. We provide bespoke technical solutions and end-to-end tracking services, empowering clients to create more market-competitive innovative products.
Welcome to contact Green Spring Technology at helen@greenspringbio.com or WhatsApp: +86 13649243917 to obtain complimentary samples, detailed product documentation, and the latest quotation information. Our specialist technical team stands ready to deliver tailored solutions, collaborating with you to expand the innovative applications of hyaluronic acid.
参照
【1】zhang k, jian j, 張z p .構造上の研究の進捗状況,特性,modi- fication and application of hyaluronic 酸か[J]。 ポリマー 公報」を発表 2015年9:217 226−です
[2] jeon o, song s j, lee k, et al。机械的强度 架橋ヒアルロン酸ハイドロゲルの分解挙動at various cross-linking 密度[J]。 炭水化物 2007年高分子 70(3): 251−257。
[3] 船尾GコーガンŠOLTES L, R,らヒアルロン酸:幅広い生物医学的および工業的用途を持つ天然の生体高分子[j]。バイオテクノロジー手紙、2006年、29(1):17−25。
[4] cowman m k, lee hg, schwertfegerk l, et al。生物学的なヒアルロン酸の含有量とサイズ アジソン病だと組織か[J]フロンティアの免疫2015年(6):261。
[5] hlavacek m .カーティルによる滑膜液濾過の役割-滑膜関節- iの潤滑における年齢。滑膜流体の混合モデル[j]。生物力学紀要進むと1993年1145 26(10):−1160た
[6] KをP MAłGORZATA K JACEKらの オキシダット-変形性関節症患者におけるive性ストレス疾患の発生に発生する可能性のある補償効果の評価の試み- nt [j]。2019年Medicina 55(5): 150騎だった
【7】 ^ a b c d e f g h i g h i g h i g h i g h i g h 酸の誘導体とその治療 効果 on 、炷き 上皮 surgical 傷 and 慢性創傷:ランダム化con trolled試験の系統的レビューおよびメタ分析[j]。2012年(平成24年)3月31日:ダイヤ改正。
[8] hotamisligilg s . inflammation, metaflammation and im- munometabolic disorders[j]。自然、542(7640)、2017年177 185−
[9] kojouharov h v, trejo i, chen b m .骨折治癒における炎症のef fectsモデル化[c]//アメリカ Insti - tute 物理学の 会議 シリーズ。 American 研究所 』文藝春秋、2017年。
[10] グリシュマs pローハンbチャールズd e数値 - vestigation 白血球は回し続ける 癒着 や債券 形成 様々なp-セレクチン密度でコーティングされた表面[j]。」。cell press(2019年). 2019年3月16日閲覧。
[11] ^ a b c d e f g h i ^ a b c d e f g h移行前 in- flammation to proliferation: a critical step during wound healing[j]。セルラー and Molecular 生活 科学: 2016年Cmlsは、73(20):3861−3885。
[12] john ch w y, abatangelo g .ヒアルロン酸の機能— a in wound repair[j]。1999年傷口修理再生7 (2): 79−89歳です
[13] hui e, gimeno k i, guan g, et al。時空間 con—phototunable hyaluronic acid hydrogelsにおける粘弾性の制御[j]。2019年生体高分子20(11日):4126と−4134。
-
Prev
Green Spring Technology's Premium Hyaluronic Acid Enhances Health Product Solutions
-
次
Exploring Hyaluronic Acid Upgrades for Eye Health Product Solutions


 英語
英語 フランス
フランス スペイン
スペイン ロシア
ロシア 韓国
韓国 日本
日本