皮膚へのヒアルロン酸の使用は何ですか?
ヒアルロン酸ヒトの皮膚組織や結合組織を含む様々な組織に広く存在する一般的な多糖類です。1934年にmeyerらによって最初に牛の硝子体液から分離されて以来、ヒアルロン酸の様々な機能が発見されてきました。その親水性、粘弾性、生体適合性、免疫原性の欠如から、ヒアルロン酸は局所薬物送達システム、創傷治癒、疾患に対するバリア療法、炎症性疾患の治療に応用されています。
1物理的および化学的性質
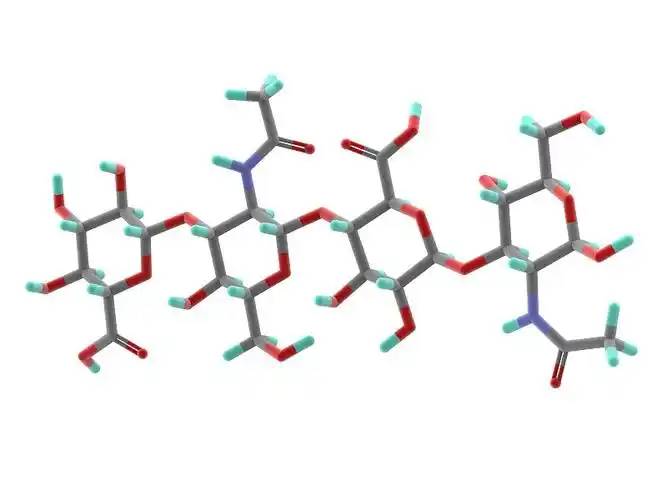
Hyaluronic acid is のlinear polysaccharide polymer composed のD-glucuronic acid and N-acetylglucosamine, with のmolecular formulの(C₁₄H₂₁NO₁₁)_n. Based on its molecular weight, hyaluronic acid can be classified inにhigh-, medium-, and low-molecular-weight types. Most のthe properties のhyaluronic acid are related to its molecular weight. High-molecular-weight hyaluronic acid (HMW-hyaluronic acid) hとしてのmolecular weight range の103–104 kDa, medium-molecular-weight hyaluronic acid has a molecular weight range of 200–103 kDa, and low-molecular-weightヒアルロン酸 (LMW-hyaluronic acid) has a molecular weight range of 10–200 kDa. Through hyaluronidase, mechanical force, oxidative stress, and other regulatory processes of hyaluronic acid degradation and metabolism, high-molecular-weight hyaluronic acid can be degraded into hyaluronic acid polymers (またはfragments) of different sizes. Low-molecular-weight hyaluronic acid can also be de novo synthesised by hyaluronic acid synthase during inflammatory processes [1]. In the human body, hyaluronic acid typically exists でa high-molecular-weight form with a size of approximately 104 kDa. It can bind to water equivalent to 1,000 times its own weight through hydrogen bonds [2] and achieve moisturising effects by reducing water evaporation and inducing hydration of the stratum corneum.
2生物特性
2.1生体適合性
Unlike collagen, which is highly allergenic, hyaluronic acid lacks antigenic epitopes [3], making it safer as a topical drug matrix. Another advantage of hyaluronic acid is that it can be rapidly degraded by hyaluronidase when adverse events occur [4]. Different molecular weights also influence the biological properties of hyaluronic acid. High-molecular-weight hyaluronic acid has higher viscosity, longer retention time, and better biocompatibility; low-molecular-weight hyaluronic acid exhibits the opposite characteristics [5].
2.2組織修復効果
When inflammation occurs or trauma forms, topically applied high-molecular-weight hyaluronic acid binds with its receptor CD44 to form a hyaluronic acid-CD44 complex, which may trigger a series of reactions でfibroblasts, such as changes in the cellular cytoskeleton and regulation of the tissue healing process [6]. During trauma, hyaluronic acid promotes fibroblast migration to the wound site via CD44 expression on fibroblast cells, thereby facilitating tissue healing. However, the receptors of small-molecule hyaluronic acid differ from those of large-molecule hyaluronic acid, and thus this 効果is not observed.
Small-moleculeヒアルロン酸チロシンリン酸化を本格的に推進できる発動phospholipase PLCγ1タンパク質を活性化させ、ナットウキナーゼC (PKC:)シグナル伝達経路およびこのmitogen-activatedタンパク質キナーゼ(MAPK)シグナル伝達システム血管の内皮細胞推進体細胞分裂血管新生を促進、容易に細胞修復[6]。
Komorowicz et al. 【7】 reported that hyaluronic acid with molecular weights of 500 kDa and 1500 kDa caused fibrin to form lateral cross-links rather than branches, thereby altering the fibrin aggregation pattern and inhibiting fibrinolysis; furthermore, on the surface of fibrin clots, 500 kDa and 1500 kDa hyaluronic acid significantly inhibited tissue plasminogen activator (tPA)-catalysed plasminogen activation; therefore, in tissue injury and inflammation, hyaluronic acid can stabilise fibrin by altering its structure and solubility.
2.3 炎症に対する調節効果
In most studies, high-molecular-weight hyaluronic acid exhibits anti-inflammatory effects when applied topically, while lower-molecular-weight fragments exhibit pro-inflammatory effects. This is primarily due to different receptors on the セルsurface for hyaluronic acid of different molecular weights, with high-molecular-weight hyaluronic acid primarily binding to CD44 and lower-molecular-weight hyaluronic acid primarily binding to toll-like receptors (TLR) [5].
High-molecular-weight hyaluronic acid binds to CD44 to form a hyaluronic acid-CD44 complex. The signal transduction cascade triggered by CD44 includes PI3K, PDK1, AKT, and the Ras phosphorylation cascade involving RAF1, MEK, and ERK1/2, thereby reducing inflammatory responses and inhibiting the production of reactive oxygen species (ROS). When inflammation occurs, the production of hyaluronic acid synthase increases, leading to enhanced de novo synthesis of small-molecule hyaluronic acid. Concurrently, under the influence of ROS or enzymatic actions such as hyaluronidase, more large-molecule hyaluronic acid is degraded into small-molecule hyaluronic acid. However, simultaneously, CD44 regulates inflammatory responses by downregulating TLR-4 expression. During this process, as large-molecule hyaluronic acid is degraded, ROS are also cleared, exerting an antioxidant effect. Additionally, hyaluronic acid regulates the expression of inflammatory cells, antigen-presenting cells, dendritic cells, and macrophages at the site of inflammation through CD44, exhibiting anti-inflammatory effects [8–11].
In the interaction between small-molecule hyaluronic acid and TLR, TLR-2 and TLR-4 activate NF-κB protein through Myeloid Differentiation Factor 88 (MyD88)-dependent and MyD88-independent pathways, exhibiting pro-inflammatory effects. In the MyD88-independent pathway, hyaluronic acid increases the expression of interferon-induced pro-inflammatory genes through type I interferon [9].
3临床応用
3.1局所薬物送達システム
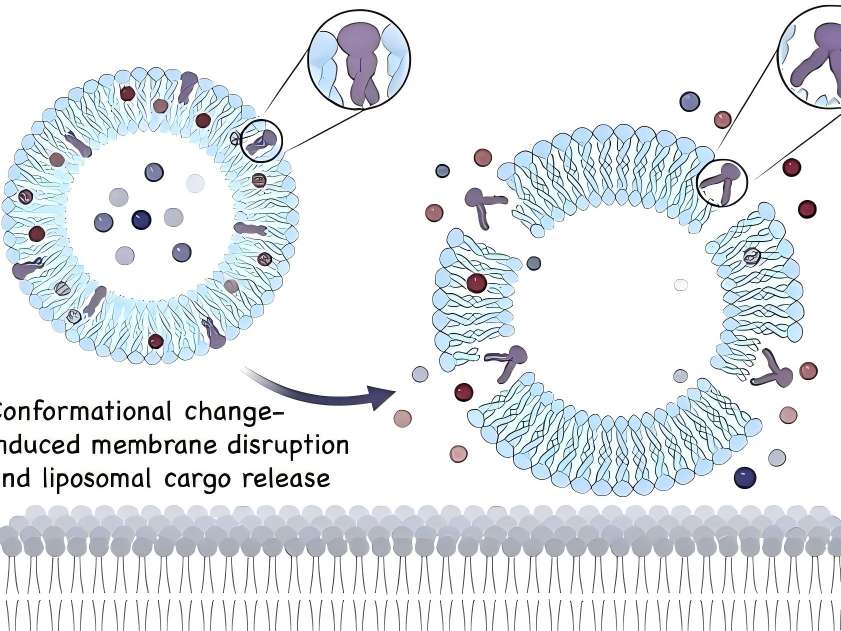
In topical drug formulations for skin diseases, liposomes containing hyaluronic acid are present on the skin surface. Hyaluronic acid can act as a mucosal adhesive agent when the drug takes effect or is absorbed. Additionally, as a topical drug formulation matrix, hyaluronic acid can prolong drug retention time [12].
フリードリヒ・et al. [13] conducted experiments on regulating inflammatory responses associated with acute injury. The combination of high-molecular-weight hyaluronic acid gel with a monoclonal antibody that antagonises TNF-α influenced the bioavailability of the monoclonal antibody, prolonging the drug&#炎症部位での39の作用時間、薬物の滞留時間を増加させ、それによって創傷治癒をより効果的に促進する。ここでは、それは薬物吸収を助けるために粘膜接着剤として機能するだけでなく、薬物を変化させる' s在留期间。より多くの薬剤で、ヒアルロン酸のそのような効果がさらに解明されるかもしれません。
3%のジクロフェナクゲルと2.5% high-molecular-weight hyaluronic acid as the matrix (trade name: Solaraze) is increasingly used in the treatment of actinic keratosis. In vitro Franz cell studies showed that compared with the buffer solution control group, after 7 days of treatment with hyaluronic acid formulations, a higher percentage of diclofenac remained in the epidermis (41% vs 25%) [14]. Clinical applications demonstrated good efficacy in clearing skin lesions, with the most common adverse reactions being contact dermatitis, skin dryness, rash, and epidermal peeling; no severe adverse reactions were reported [15].
Advances in nanotechnology have made the application of new technologies possible, such as hyaluronic acid-based nanocapsule polymers, which have shown promising efficacy as a novel topical drug delivery matrix in experimental studies. Compared with non-polymeric micelle solutions containing similar drug concentrations, in vitro skin penetration analysis showed that after 5 hours of local drug application, the drug concentration in the epidermis increased by 3 times, while the drug concentration in the dermis increased by 6 times. Additionally, hyaluronic acid polymer micelles enhanced the drug' s bioactivity[16]。
3.2傷口がふさがる
考慮hyaluronic acid promotes tissue repair, it can shorten wound healing time and reduce scar formation. Many clinical studies have reported such effects of hyaluronic acid. In the healing of 21 wounds caused by erbium laser, the wounds of subjects treated with oat Rhealba extract and high-molecular-weight hyaluronic acid formulations healed within 9 days, while the control group treated with panthenol and hydroxyproline formulations also healed within 9 days. while those treated with resveratrol copper healed in 12 days, and the untreated control group healed in 16 days. The hyaluronic acid group showed a significant reduction in healing time [17]. In a clinical trial involving 60 cases of local burns (average burn area approximately 3% of body surface area) treated with a high-molecular-weight hyaluronic acid zinc gel, wound size reduced to 50% of the original wound size by day 5, 93.3% of participants achieved complete epithelialisation by day 21, and 91.7% of participants experienced pain relief by day 10. No wound infections occurred during the wound healing process [18].
In a controlled experiment involving 30 cases of second-degree burn healing, olive oil and high-molecular-weight hyaluronic acid had similar effects on burn healing time, but olive oil was more effective in preventing scar formation [19]. In a randomised clinical trial comparing the topical application of high-molecular-weight hyaluronic acid and a neutral medium on 89 cases 足も静脈ulcers, the results showed that by day 45, the ulcer healing area was significantly larger in the hyaluronic acid group compared to the control group (hyaluronic acid group: 73.4 ± 4.6% vs. control group: 46.9 ± 9.6%, P = 0.011), and the number of healed 潰瘍at 45 and 60 days was significantly higher than that in the control group (45 days: hyaluronic acid group 31.1% vs. control group 9.3%, P = 0.011) (60 days: hyaluronic acid group 37.8% vs. control group 16.3%, P = 0.024) [20].
3.3 病気のためのバリア療法
Studies have shown that impaired epidermal barrier function plays a significant role in the development of atopic dermatitis and other allergic diseases, and dry skin is more prone to eczema. The addition of moisturisers containing hyaluronic acid can improve the integrity of the stratum corneum. Some studies have shown that barrier therapy can reduce the frequency and severity of skin disease flare-ups and decrease the need for topical corticosteroids or topical calcineurin inhibitors. Among these, hyaluronic acid may play a crucial role in the epidermis'外傷に対する自然反応(アトピー性皮膚炎におけるケラチノサイトの移動、創傷治癒およびバリア修復時の細胞増殖など)[21]。
Palmer et al. [22] conducted a randomised controlled trial to evaluate the efficacy and 安全of MAS063D (a cream containing high-molecular-weight hyaluronic acid) in treating 30 patients with atopic dermatitis. After two weeks of treatment, MAS063D significantly reduced the itch severity index (EASI) in patients; Draelos et al. [23] conducted a controlled trial comparing a 1% high-molecular-weight hyaluronic acid-based pimecrolimus gel with a medicinal ceramide cream in the topical treatment of atopic dermatitis. At the start of the experiment, and at weeks 2 and 4, patients were assessed for erythema, crusting, lichenification, skin peeling, itching, stinging, and burning. At week 2, patients in the hyaluronic acid group showed significant improvement in eczematous lesions compared to the control group, but there was no significant difference in lesion recovery by week 4. Hyaluronic acid-based formulations are easier to apply and absorb than traditional drugs and have a milder odour, improving patient compliance. However, this study had a small sample size, no control group was established, and an appropriate assessment sy幹was lacking, necessitating further experiments to support these results [24].
3.4の炎症性疾患
The anti-inflammatory regulatory mechanisms of hyaluronic acid have been demonstrated in some drug delivery media, skin barrier function repair, and wound healing. Additionally, topical hyaluronic acid has shown efficacy in treating facial seborrheic dermatitis. However, in in vitro experiments on inflammation regulation, small-molecule hyaluronic acid often exhibits pro-inflammatory effects (as described in Section 2.3), so the mechanism of topical hyaluronic acid in treating inflammatory diseases remains unclear. The main drugs used for facial seborrheic dermatitis are corticosteroids and antifungal agents, but their application is limited due to adverse reactions and drug sensitivity issues. A clinical trial by Schlesinger et al. [25] showed that topical application of 0.2% sodium hyaluronate improved symptoms such as erythema and itching in patients with facial seborrheic dermatitis. However, this clinical trial had a small sample size, and whether small-molecule hyaluronic acid regulates inflammation in a positive or negative manner remains controversial, so further clinical studies are needed to confirm its efficacy.
4結論
Topical hyaluronic acid is safe and effective in dermatology. With the maturation of bacterial fermentation methods for ヒアルロン酸の生成また、バイオ安全性がさらに向上し、従来の方法に比べて製造コストが削減されています。これにより、局所適用に適しています。バイオテクノロジーの継続的な発展に伴い、ナノ粒子ポリマーミセルのような新素材の出現は、皮膚科での局所使用のためにヒアルロン酸がより優れた物理化学的特性を示すことを可能にするかもしれない。
In many skin lesions, such as psoriasis, the normal hyaluronic acid reticular structure is partially missing in the spinous layer and granular layer, and hyaluronic acid is present in the stratum corneum of dyskeratosis. Whether correcting the abnormal distribution of hyaluronic acid in the epidermis of psoriasis patients can improve the symptoms of related skin diseases is worthy of further study. Studies have shown that with the increase of age, the expression of hyaluronic acid and CD44 receptor in the skin showed a downward trend. At this time, the role of exogenous hyaluronic acid in various aspects was weakened, so whether there were differences in the dosage and frequency of use at different ages is still worth discussing. Because hyaluronic acid exerts its antioxidant effect through its own degradation and ROS elimination mechanism, its application in anti-aging drugs is worth tracking.
参照
[1] 梁J、姜 D、貴族 PW。Hyaluronan as a 治療 対象人類 病か[J]。Adv 麻薬 Deliv 牧師さん、2016年で 97: 186-203。
[2] stern r, asari aa, sugahara kn。ヒアルロン酸断片:情報に富んだシステム[j]。^ eur j cell biol,2006,85(8): 699-715。
[3] Nowacki M, Pietkun K Pokrywczyńska 充填効果、持続性、および safety 真皮の sn-bi系ハンダ 制定と stem 細胞 動物モデルでは[j]。Aesthet Surg J 、2014年、34(8):1261-1269人である。
[4] delorenzi c .ヒアルロニダーゼによるヒアルロン酸フィル- erの経動脈分解[j]。^『仙台市史』通史編(通史編)、832頁 841.
[5] zhao n, wang x, qin l,et al 細胞増殖とos- teogenic分化におけるヒアルロン酸の濃度 in 体外か[J]バイオケミカル Biophys 「Res publica 2015年Commun、465(3):569-574。
[6] slevin m, kumar s, gaffney j .血管新生オリゴ糖 of hyaluronan 誘導 複数 シグナリング 経路 影響 血管内皮細胞のmitogenic and wound healing respon- ses[j]。^ a b c d e f g h i j biol chem,2002,277(43): 41046-41059。
[7] komorowicz e, balazs n, varga z,et al 機械的安定性を低下させるが、フィブリン行列の溶解性re- sistanceを増加させる[j]。^ a b c d e f g h i l l、2017年、55-68頁。
[8] petrey ac,de la motte ca .炎症の重要な調節因子であるヒアルロン酸[j]。^アポロドーロス、2014年、5巻101。
[9] naor d .編集:ヒアルロン酸とその受容体との相互作用 (RHAMM CD44) 調整 the 活動 炎症やがんの原因になります[j]^パウサニアス、2016年7月7日、39頁。
[10] vigetti d, karousou e, viola M, et ヒアルロン酸:生体合成とシグナル伝達[j]。2014年Biochim Biophys Acta、 1840年(8年):2452-2459。
[11] kim y, lee ys, hahn jh,et al。ヒアルロン酸ターゲット cd44はpkcdel - ta、rac1、ros、および関連するfcepsilonriシグナル伝達を阻害する MAPK to 発揮 出て effect [J]。^ a b c d e f g h i『人事興信録』第45版、2537-2547頁。
[12] law ch, li jm, chou hc,et al。ヒアルロン酸依存性- ent 保護 in H9C2 cardiomyocytes: a cell モデル 心臓の虚血再灌流損傷と治療[j]。^『川崎市史』、川崎市、2013年3月、54-71頁。
[13] Friedrich フホウ譜ミッチェル・リヴィングストン・ウォッシュバーン NR.Transport パターン of 反 やけどTNF -α貼る:治療意味合いをhyalu - ronic酸共役[J]。2017年生体材料、114:10-22。
[14] brown mb, hanpanitcharoen m, martin gp。in vitroで-皮膚上のグリコサミノグリカンの効果への痕跡 溝 宣誓証言 of 非ステロイド性抗炎症[J]。Int J Pharm、 ^『官報』第2121号、大正2年(1913年)12月1日。
[15] gupta ak、paquet m、villanueva e、et al actinic keratoses [J]。コクラン データベース Syst 2012年牧師、 12.
[16] mejkalova D、Muthn∮T, K Neporova et al.Hyaluronan高分子micelles外用薬物伝達か[J]。^ a b cアポロドーロス、2017年、156 -96頁。
[17] sabadotto m、theunis j、black dらin vivoでのavena rhealba含有クリームの効果評価( )エルビウムyagで生成された脱表皮肌で皮膚barri- erの回復にエキスとヒアルロン酸 レーザーか[J]eur j dermatol,2014,24(5): 583-588。
[18] juhasz i、zoltan p、erdei i . zn-haによる部分的厚さ火傷の治療:臨床試験研究の教訓[j]。^ a b c d e f g h『仙台市史』第2巻、2012年、82-85頁。
[19] campanati a, de blasio s, giuliano a,et al.部分的toの治療のための局所オゾン化オイル対ヒアルロン酸ゲル full-thickness 火がついて バーンズ: 前向き、com- parative、単盲、非ランダム化、対照臨床 2013年裁判[J] .Burns、39(6):1178-1183。
[20] humbert p, mikosinki j, benchikhi h,et al 治療中のヒアルロン酸を含むガーゼパッドの安全性 of leg ulcers of venous or 混合起源: a double-blind、 randomisedする 裁判か[J]。Int 傷 2013年J、10 (2): 159-166。
[21] maytin ev, chung hh, seetharaman vm。ヒアルロン酸parは、in vivoでper- meabilityバリアの破壊に対する表皮反応に発現する[j]。^ a b c d e f g h i(2004年)、165頁。 1331-1341。[22] palmer cn, irvine ad, terron-kwiatkowski a,et al.com - mon表皮バリアの機能喪失変異体-テイン・フィラグリンはアトピー性皮膚炎の主要な素因因子である[j]。^ a b c d e f g h i(2006)、441-446頁。
[23] Draelosツンデレ。同等の有効性の臨床評価 ヒアルロン酸ベースのフォームとセラミド含有eマルションクリームの軽度から中等度のアトピーの治療 皮肤炎[J]。j cosmet dermatol,2011,10(3): 185-188。
[24] frankel a, sohn a, patel rv,et al.ピメクロリムス1%クリームとアトピー性皮膚炎患者の治療におけるセラミドヒアルロン酸エモリエントフォームの両側比較研究[j]。^ a b c d e f g h i j drugs dermatol,2011,10(6): 666-672。
[25]やがてシュレージンガーTローランドPC顔面脂漏性皮膚炎の治療における低体重ヒアルロン酸局所ゲルの有効性と安全性[j]。J Clin Aesthet 2014年Dermatol、7(5):15 ~ 18。


 英語
英語 フランス
フランス スペイン
スペイン ロシア
ロシア 韓国
韓国 日本
日本




