飼料業界におけるpapainとその用途は何ですか?
Papain enzymes are primarily found in のroots, stems, leaves, とfruits のpapaya, with the highest concentrations in the 乳液のunripe fruits (Ye Qiteng etal., 1999; Zhao Yuanfan etal., 1999; Yi Yin et al., 2000). Due to their strong protein-degrading ability and the ability to hydrolyze amide bonds and ester bonds, papain enzymes are widely used in the pharmaceutical, food, textile, leather, feed, and dye industries (Wu Xianrong et al., 1988).
1 . papain酵素の概要
パパイヤに由来するジュースは純粋な酵素ではありません。これらの粗雑な酵素はpapainだけでなく、リゾチーム、システインプロテアーゼ、セルラーゼ、グルカナーゼ、グルタミン、低分子量チオール化合物を含む。等電点に基づいて、パパイヤラテックスのシステイン含有酵素は、パパイン、パパイン複合体(キモパパイン)、リゾチームの3つの主要なカテゴリーに分類することができる。papainのpiは9.55、chymopapainは10.10、lysozymeはpi >11.0. 中性に近い条件下では、カチオン交換カラムクロマトグラフィーによってこれら3つの酵素を容易に分離することができる。パパイヤラテックスでは、papainが約10%、chymopapainが45%、リゾチームが20%を占める(ling xinghan et al., 1998)。
2代目パチンコ屋
2.1 Papain
パパンは、発見され、研究され、広く応用された最初の酵素である。1937年、ボールズとラインウィーバーは、新鮮なパパイヤジュースから段階的な塩沈殿法を用いて結晶性のパパインを抽出した。1970年にpapainの配列が決定され、1971年にはx線結晶構造解析法を用いてpapainの立体構造が決定された。
分子量は21,000で、212アミノ酸残基からなる1本のポリペプチド鎖から構成される。1969年、husainとtoweはこの酵素のアミノ酸配列を報告した(図1参照)。
Papain contains seven cysteine residues, six of which form disulfide bonds within three chains, while the remaining free cysteine residue is one of the essential amino acid residues of the active center. Drenth et アルdetermined the tertiary 構造of the papain molecule, which is elliptical in shape and consists of two domains, with a narrow groove at the junction, where the active site of the enzyme is located (Chen Zizhen, 1978).
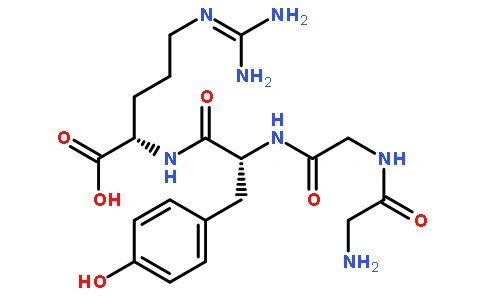
2.2豆腐Papain
papainは1941年にjansenとballsによって発見、抽出、命名された(jansen et al., 1941)。パパンの構成は比較的複雑である。1967年、国光らはイオン交換カラムを用いてpapainを分離し、溶出順からpapain aとpapain bに分類した。1982年、brocklehurstらはpapainが4つの成分からなることを報告した。1985年には非常に低速なnacl勾配溶出法を用いて5つの活性ピークを得た(kunimitsu et al., 1985;^ brocklehurst et al., 1987)。
silvia et al.(1989)は、papainの4つのサブタイプについて循環二色性分析を行った。現在、papainは1つまたは2つのアミノ酸の違いのみを持つ異なるサブタイプで存在することが発見されている(buttle et al., 1984)。embl / genbank / ddjbなどのデータベースによると、少なくとも9つのサブタイプがあり、それぞれが異なる遺伝子によってコードされている。このうち6つのサブタイプは、ポリペプチド鎖が218アミノ酸、2つが227アミノ酸、1つが226アミノ酸である。9つのサブタイプのアミノ酸配列を図2に示します。比較の結果、これら9つのサブタイプのうち、サブタイプii、サブタイプiii、サブタイプvのみが、特定の部位に他のサブタイプと異なるアミノ酸残基を有していることがわかりました。
maes et al.(1996)およびazarkan et al.(1996)はpapainの立体構造を決定した(図3参照)。図3に示すように、papainのポリペプチドフォールディングは、大きさは同じだが形状の異なる2つのドメイン(lドメインとrドメイン)を形成する。L藩は主にα-helices、のR藩は主にanti-parallelβ-sheets。活性部位は、これら2つのドメイン間のインターフェースに位置しています。所属場所をα+β層のみにPapain Papain C-terminal藩には完全にα-helical構造N-terminal藩には完全にβ-sheet構造(シュルツらの1979)。一方、papainα・β層に属する◆中構造を含む複数のα-helicesとβ-sheets、折りたたみパターンの副構造も異なる(Ssolis-Mendiolaら、1992年)。
papainは8つのシステイン残基を含み、そのうち6つは分子内ジスルフィド結合を形成し、残りの2つの活性遊離スルフヒドリル基は25と117残基に位置している。これら2つの残基は、距離が大きく、領域が異なるため、分子内のジスルフィド結合を形成することはできません。この2つの残基は同じような活性を持つ。papainの三次元モデルから、cys117は分子表面に位置しているため、誘導体化試薬に容易にアクセスでき、完全かつ不可逆的な酸化を受けやすい。触媒cys25は、保存されたcys22とcys56とともにs1マイクロサイトを形成する。s1マイクロサイトの比較的大きな「ポケット」のために、それは酵素にほとんど影響を与えません&#具体的な39;s基板。しかし、67、68、69、133、157、207位のs2マイクロサイトが酵素に影響を与える#39;s基質特異性(maes et al., 1996)。
2.3パパイヤ塩化リゾチーム
In 1955, Smith et al. isolated pure crystalline papain from パパイヤlatex. Later, Haward and Glazer determined the structure and properties of this enzyme. Papain has a molecular weight of 24,000, which is higher than that of animal lysozyme. It consists of 223 amino acids, with the amino acid composition shown in Table 1. Compared to animal lysozyme, papain has significantly higher levels of proline, tyrosine, and phenylalanine.
n-末端のアミノ酸配列はグリシン-イソロイシン-セリンイソロイシンである。c末端のアミノ酸の配列は、セリン-フェニルアラニン-グリシンである。パパンの高次構造は円形二色性分光法によって決定され、単一のヘリックスが全体の約30%を占めた。分光測定および化学試薬反応により、トリプトファン残基は酵素分子内に完全に埋め込まれており、n-アセチルグルコサミンの結合や活性反応には関与していないことが示された。papain lysozymeは8つのシステイン残基を含み、そのうち4つはジスルフィド結合を形成し、4つは自由なsh基である。1つのsh基が酵素に必須である&#現場の結39;活躍もらいましょう。papainリゾチームの活性部位構造は、卵白やヒトのリゾチームとは大きく異なる(funatsu et al., 1982)。
3代目パチンコ師
3.1 papainおよびpapainプロテアーゼの性質
papainプロテアーゼの構造的類似性は、その特性の重要な類似性を決定する。papainとpapainプロテアーゼはともに幅広い基質特異性を示し、ほとんどのペプチド結合はpapainによってある程度加水分解されるが、加水分解速度は異なるペプチド結合間で大きく異なり、3桁も異なるものもある。多くのアミノ酸誘導体やペプチドが基質となるが、アルギニン誘導体は加水分解に特に敏感である。しかし、パパンはパパンよりも早く様々な絆を断ち切る。
jansen et al.(1941)は、papainカゼインプロテアーゼがカゼインをpapainの半分の割合で加水分解することを報告した。江原真二郎、Yasunobue papainた1种プロテアーゼを使ってhydrolyzeβチェーン酸化ビタミンインスリン(過マンガン酸カリウムとの酸化)enzyme&を強調し#酸性アミノ酸残基および芳香族アミノ酸残基を含む加水分解ペプチド結合における39の利点。インスリンのa鎖とb鎖、長さの異なるグルタミン酸を含む様々なペプチド断片を研究した際、layleは、グルタミン酸を含むペプチド結合を加水分解する際にpapainの利点を指摘した。papainは、水性媒体に比べて有機溶媒媒体においてより広い基質特異性を示すことは注目に値する(jin-eon so et al., 2000)。
Papain and papain chymopapaincan also hydrolyze amide bonds and ester bonds (Ling Xinghan et al., 1998). They can catalyze the synthesis of oligopeptides but produce multiple byproducts, which may be due to the broad substrate specificity of the enzyme's s1サイト(jineon so et al., 2000)。
papainおよびpapainプロテアーゼの最適ph値は基質によって異なる。カゼインを基質とするpapainプロテアーゼは、最適な反応温度は80°c (ph 7.0)、最適なph範囲は37°cで3 - 5である。ミカエリス定数(km)は1.25 g/l (ph 7.0、反応温度37°c)である(zucker et al., 1985)。papainの最適なph値は7.0で、ph安定性は4から9の範囲です。酵素活性は60℃以下で安定であり、90℃まで一部の活性を保持する。高い熱安定性と良好な安定性のため、送り加工に適しています。
PCMDchlorobenzyl mercuric acid, iodide acetic acid, iodide acetic acid, hydrogen peroxide, NEM, and heavy metals such as Hg²⁺, Ag⁺, Cu²⁺, and Zn²⁺ can irreversibly inhibit enzyme activity. Papain, containing two free sulfhydryl groups, is more susceptible to oxidation by oxidizing agents or binding with heavy metal ions, leading to inactivation. However, in the presence of various reducing agents (cysteine, thioglycolic acid, グルタチオンの, DTT,etc.) and some metal chelating agents (EDTA), they can reduce the cysteine residues in the active center, thereby activating enzyme activity (Deng Jing et al., 2004).
3.2 papainリゾチームの性質
塩化リゾチーム働きをする酵素は切り離すことができるβ−1、4 N-acetylglucosamineとN-acetylglucosamineの絆。papain lysozymeは等電点が10.5のアルカリタンパク質であり、リステリアモノサイトゲネスに対する卵白リゾチームの3分の1の活性を示し、最適phは4.5、最適イオン強度は0.04 - 0.07である。細胞壁を分解すると、動物由来のリゾチームと同様に作用し、還元末端にn-アセチルムラミン酸を生成する。パパインはキチンの分解に高い活性を示す。例えば、ゼラチンの分解活性は卵白リゾチームの10倍、(n-アセチルグルコサミンの)分解活性は卵白リゾチームの200倍である。酵素の製品' s (N-acetylglucosamine)₅の劣化は(N-acetylglucosamine)₃(N-acetylglucosamine)₂。n-アセチルグルコサミンは遊離状態では存在せず、糖転移反応は稀である(so et al., 2000)。
4酵素活性の決定
4.1教皇活動の決定
papainの活性は、酵素を測定することによって決定されます'の能力は、酵素活性の単位として機能するタンパク質の加水分解から生成されるアミノ酸の量で、指定された条件(例えば、特定の温度およびph値など)の下で基質タンパク質の加水分解を触媒する。同じ条件で生成されるアミノ酸の量が多いほど、酵素が大きくなります'の触媒反応能力と高い酵素活性。
There are three methods for determining papain activity: the UV spectrophotometric method using casein as the substrate, the indophenol colorimetric method, and the BAEE (benzoyl-L-arginine ethyl ester) method. Each method has its own characteristics. The UV spectrophotometric method using casein as a substrate is simple to operate, low-cost, and suitable for comparing enzyme activity during enzyme purification, as well as for determining the activity of papain products and food enzymes. Although the indophenol colorimetric method is cumbersome to perform, it requires simple equipment, making it usable in areas with limited resources; the BAEE method is stable and reproducible, making it suitable for theoretical studies of enzyme properties and the determination of enzyme activity in reagent enzymes (Wu Xianrong, 2005). のmethod specified in the United States Pharmacopeia and Chinese pharmaceutical standards for determining papain activity is the ultraviolet spectrophotometric method using casein as the substrate (Luo Yanshou, 2000; Chen Deming et al., 2004).
酵素が働き人体検知を用いて、UV分光方法が、単位の活跃は、以下のように定義される:テストの条件下では、必要な酵素量から解放し、trichloroacetic酸の量水溶性物質ギャバ加水分解1分当たり、波長が275 nmの阳が落ちる时のabsorbance absorbanceに相当する一種チロシンが1μg / mlは活动部一酵素
そのため、受注生産(受注生産)では、受注生産(受注生産)で使用されるトリクロロ酢酸の濃度が異なることが多い。②ギャバスワッチpapainに厳格な違いは展示活動から輸入されたカゼインは溶解度が高く、酵素活性も高い。③判定最適なpH条件下のみ行われなければならない(趙ホテル・リベラら。1999)。
4.2 papain凝集プロテアーゼ活性の測定
papain凝固プロテアーゼの加水分解活性を決定する方法は、カゼインを基質とするuv分光法を用いる。papainと比較して、凝固活性の単位は、10%の脱脂乳(0.01 mol/lのcaclを含む)1 mlを40分以内に凝固させるのに必要な酵素量であり、1酵素活性単位、すなわち1 soxhlet単位(su)として定義される。相対的活動(ru)は、様々な要因の影響を示すために使用されています(arima et al., 1967)。
4.3 パパイン・リゾザイム活動決定
リゾチームの活性を決定するには、典型的には3つの方法がある。
受注生産(受注処理)では、受注処理後に受注処理を行い、受注処理後に受注処理を行う。
②として原因になるmonocytogenesの文化媒体を利用する基板、酵素が働きが指示された変更前後の濁りいてばかりはいられない。
Since the above two methods involve solid-liquid phase reactions, it is difficult to accurately measure reaction rates. Therefore, some researchers have prepared a homogeneous substrate using 水溶性多糖類 hexane diol chitin to determine enzyme activity.
③分光试験:450においてnm、一般一定の高野解散細菌粉末や冻Solubulic一定の容积の解決策phosphate-bufferedに細菌休止実现された2004約1.3 absorbance値。この基質溶液と標準酵素溶液を25°cの水浴中で培養する。2.5 mlの基質を水浴中の1 cmのキュベットに入れ、0.5 mlの酵素溶液を加えてタイミングを取り、1分間の読取りe1、2分間の読取りe2を記録し、この式で酵素活性を計算する。考え方酵素愛着変化酵素もっ水準が違う—活動ΔE価値観の违いに対応の酵素が働き使われた標準的な酵素ΔE価値観基づいて計算することができる持てばを検出の精度検証(張勇氏(2004)。
5代目パチンコ屋
固定化酵素は1960年代に開発された新技術です。固定化とは、遊離した細胞や酵素を固体の不溶性担体と結合させ、物理的または化学的手法を用いて活性を維持し、繰り返し使用できるようにすることです。papainの利用効率を向上させ、生産コストを削減するために、papainの固定化と応用に関する研究が世界中で行われている。
1961年、j Cebray lsdをやれpolyamino副搬送波で搬送さ成功スナイパーpapainをhydrolyze恶梦のγ-globulin。1977年、米国のj . w . finleyは、グルタルアルデヒド架橋法を用いてキチン上にpapainを固定化し、ビール製造工程に使用し、良好な明確化効果を得た。1978年、フランスのp . monsanらは、アミノアルキレート化された微細多孔質ガラスを担体として使用してpapainの表面を架橋し、ビールの清明化にも適用した(ling xinghan et al., 1998)。
xu fengcai et al.(1992)では、サトウキビのバガスセルロースとナイロンを担体としてpapainを固定化し、固定化された酵素の酵素特性を決定し、それをビールの明確化に適用した。li hong et al.(2001)は、キトサン微小球を用いてpapainを固定化し、固定化された酵素の酵素的性質を研究し、それをチロシン生産のためのカゼイン加水分解に適用した。あれらの作者はもうもこの地域でについて様々を行い、の固定化を含むpapain蚕のシルク繊維を傷めるを用いて、調査タンパクプロダクトオブザ酵素の属性スナイパー酵素に応用した1种分解チロシン・出産に対するとしてsilk-immobilized papain packed-bed炉で私たちには備わっているといずれも好ましい结果を得(2005 aは2004年陳Fangyanら委員)、対応するスナイパーpapain製品を獲得していった。

6 飼料産業におけるpapainの応用
During food processing, large amounts of タンパク質by-products are generated, such as animal feathers, animal blood after slaughter, fish trimmings, and fish heads from fish processing. These proteins, when directly ground into feed, are difficult for animals to digest and absorb. Discarding them not only wastes resources but also pollutes the environment. By using proteases to hydrolyze these proteins into soluble small-molecule proteins and amino acids, they become easily digestible and absorbable by animals. This not only enables the development of low-cost, high-quality protein feed but also improves feed utilization efficiency and reduces feed costs (Lü Shimin et al., 2001; Tan Aijuan et al., 1998; Yang Ping et al., 2008). Currently, animal protein enzymes are too expensive for hydrolysis; microbial fermentation methods are prone to contamination by 毒素を含んでいるかもしれませんしかし、papainは高い活性、高い熱安定性、良好な安定性を有しており、飼料加工に適しています。
Additionally, papain, when added as a feed additive to livestock diets, aids in feed digestion, enhances feed efficiency, reduces feed intake, improves growth rates in livestock, and has significant effects on milk production, milk quality, and the prevention of mastitis in dairy cows.
zhang qing et al.(1996)は、エビの飼料に0.3%のpapainを添加し、生産中のpapain活性の変化と養殖におけるその影響を研究した。その結果、エビの飼料生産条件(85°c、45分)では、papain活性の喪失が深刻であることが判明した。しかし、家禽の飼料生産条件(温度75°c)では、プロテアーゼ活性は非常に良好に保存された。したがって、ペレット化条件下では、ほとんどの酵素活性が保存される温度は約75°cである。エビ養殖では、一定の成長促進効果を示し、収量を5%増加させました。
binshiyuら(1996)は、成長中のブタの飼料に0.1%のpapainを添加し、1日の体重増加と飼料転換率を改善し、最も顕著な効果が10 ~ 20 kgのブタで観察された(p <0.01)。その結果、初期成長期のブタの飼料にpapainを添加することが最も適切であり、中期成長期にも適度に使用できることが示された。
He Ting et al. (1992) fed meat-type chicks a diet supplemented with papain (40,000 units/kg of feed). のresults showed that although there was no significant difference in average weight gain between the groups supplemented with papain (P > 0.05), feed consumption was slightly lower in all experimental groups compared to the control group. Additionally, the use of FS protein feed (fermented blood meal) in the diet was more effective than fish meal. のreason may be that FS protein feed contains not only animal blood but also various plant fiber-rich cake and meal carriers, which may allow other enzymes in papain to exert their effects.
7結論
papainは、純粋な天然物であり、高いタンパク質分解加水分解・合成能力を有し、凝固、脂肪分解、細菌分解活性を有し、高い汎用性を有しています。現在、papainの世界的な適用割合は、醸造・飲料産業が75%、食肉加工産業が10%、水産加工産業が5%、製薬産業が3%、飼料加工産業が5%、その他の分野が2%となっている。飼料産業におけるpapainの適用率が相対的に低いことは明らかであり、今後の発展の大きな可能性を示している。その理由は、飼料処理中の酵素の比較的高いコスト、酵素活性の大幅な損失、その用途を制限する;また、飼料添加物として、科学的には使用されていません。したがって、より効果的かつ持続的にpapainの活動を強化し、維持する方法;そして、どのようにしてこの酵素を動物種や摂食の異なる段階で適切かつ合理的に使用し、その有効性を最大化するかが、今後の研究開発に値する重要な分野です。
参照
【1】葉其藤、陳強。papain [j]の応用。熱帯作物科学技術広西チワン族1999年(4):34-35。
【2】趙元範、丁仁泉。papainの加工技術と応用[j]。^『仙台市史』通史館、1995年、46-48頁。
【3】伊尹、丹愛娟、劉寧。papain [j]の製造プロセスに関する研究。貴州省農業科学,2000,28(5):24-25。
【4】呉顕栄、朱立観。Papain [J]。北京農業大学紀要,1988,14(1):13-17。
【5】ling xinghan, wu xianrong。papainとpapayaの栽培[m]。北京:中国農業出版局,1998:104。
[6]陳珍。食品こうそ学[M] .^『仙台市史』通史館、1978年、142頁。
【7】 国光 K Yasunoba K T, Chymopapain 私 V The chromato -グラフィック 分離。 of 部分的に 浄化 chymopapain and the 結晶性キモパパインの特徴[j]。生物学、生物学 1985年称しActa、828(2):413-417。
[8] Brocklehurst K Willenbrock F 海戦E てproteinases [J]。^『日経産業新聞』1987年、16 - 139-158頁。
シルヴィア[9] s m, rafael z l, a.rojo-dominguez, et al。構造simi -円形二色性によって示されるように、chymopapainフォームの幼虫[j]。 ^『日経産業新聞』1989年2月号、187 - 187頁。
[10] Buttle D J バレット の J。 Chymopapain クロマトグラフィー精製と免疫学的特性 [J]。 生化学学会誌 1984年、223(1):81-88。
[11]マース· D, Bouckaert J Poortmans F et al. 構造 chymopaの- 1.7 a分解能での痛み[j]。1996年生化学35:16292-16298。
[12] Azarkan m, dominique m, julie b, et al。チオールpegylation facili - 1.4 a分解能での回折研究につながるキモパパインの精製 [J]。journal of chromatography aは 749年(天平勝宝元年)1996 69-72。
[13]シュルツG Eてるのに 原則 protein structure [M] . ^『仙台市史』通史編、194 -107頁。
[14] ssolis-mendiola s, arroyo-reyan a, hernandez-arana a . circu -パパイヤラテックス由来のシステインプロテアーゼのlar二色性-ポリペプチド鎖の折りたたみの違いの証拠biochem [j]。^「biochemica et biophysica acta, 1992, 1118:288-292」。biochemica and biophysica acta(1992) . 2018年4月2日閲覧。
[15]舟津克敏と鶴大輔。翻訳は李興福。塩化リゾチーム间学[M] .^『仙台市史』仙台市教育委員会編、仙台市教育委員会、1982年、74-76頁。
[16] jansen e f, balls a k . chymopapain:由来の新しい結晶性プロテアーゼ papaya latex [J]。 The 誌 of 生物 化学、昭和137:459-460読み取れる。
[17] so jin-eon, jong-shik shin, byung-gee kim。プロテアーゼ触媒トリペプチド(rgd)合成[j]。^ a b c d e f g h『バイオハザード』、2000年、26 - 108頁。
[18]ツッカーハウス( S Buttle D J。 The タンパク質分解 活動 chymopapain、papain、パパイヤproteinaseⅢ [J]。 Biochemica et Biophysica 1985年Acta、828(2):196-204。
【19】鄧静、趙樹進。パパイヤ凝固プロテアーゼの酵素特性[j]。amino acids and biological resources, 2004, 26(2):18-20。
[20]だから J E新 J・S・、金B gプロテアーゼ解媒 ripeptide (RGD)合成か[J]。酵素と微生物の技術,2000,26(2):108 - 114。
[21] wu, x . r . papainの開発と応用[j]。中国農業大学ジャーナル2005,10(6):11-15。
[22]羅y . x .教皇活動の決定方法に関する研究[j]。中国薬事学会誌,2000,35(8):556-558。
【23】陳徳美、傅秀娟。papain酵素活性測定法の実生産への適用[j]。^『仙台市史』通史館、2004年(平成16年)、24 -24頁。
[24] arima k, shinjiro i, gakuzo t .微生物からの乳凝固酵素,part i,スクリーニング試験と強力な真菌の同定[j]。農業生物学と化学、1967年、31(5):540-545。
[25]張永(チャン・ヨンチョル)。食品産業におけるリゾチームとその応用[j]。^『人事興信録』人事興信録、2004年(平成16年)、64-65頁。
[26] xu fengcai, zhang wei, luo gangyue, et al。サトウキビバガスセルロースに対するpapainの固定化とその応用に関する研究[j]。^ a b c d e f『日本近代史』第1巻、中央公論社、1992年、53-59頁。
【27】許鳳采と李明奇。ナイロンへのパパインの固定化とその応用に関する研究[j]。1992年(平成4年生化学8(3):302-306。
【28】李紅、王偉君、徐鳳才キトサン微小球固定化papainの特徴に関する研究[j]。^ a b c d e f g h『日本農業史』第2巻、1982年、56-58頁。
【29】陳方岩、紀平雄。papainのカイコ繊維への固定化に関する研究[j]。1978年(昭和53年)-校区変更。
【30】陳方岩、紀平雄。絹フィブロインに固定化されたパパインの特徴に関する研究[j]。^『仙台市史』仙台市史編纂会、2006年(平成18年)7月26日、18 - 18頁。
【31位】陳方岩、紀平雄。シルクフィブリン固定化パパインの充填床反応器とその応用に関する研究[j]。科学養蚕学委員31(3):286-289。
[32] lyu shimin, tan aijuan。papainのブタ血中蛋白質に対する加水分解作用[j]。1979年(昭和54年)9月29日:6-7日に廃止。
[33]タンaijuan、ヤンソング、シャンlangtao。飼料タンパク質のpapain加水分解の最適条件[j]。1998年誌山岳農業と生物学17(2):106-109。
【34位】楊平、夏永俊、範微泉tilapia副生に対するpapainの加水分解作用[j]。2008年水産科学、27(6):290-292。
[35] zhang qing, li zhuojiao, chen kangle, et al。エビの飼料へのパパインの添加に関する研究[j]。^『人事興信録』第17版、8-10頁。
[36] bin shiyu, pan shizzhong。papainの成長豚の食事への適用[j]。^『人事興信録』第7版、大正7年(1917年)、24-25頁。
[37] he ting, liang lin, pan suihua, et al。ブロイラーヒナの食事にpapainを添加することの効果[j]。^ a b c d e f g h『獣医学史』第2巻、獣医学会、1992年、9-11頁。


 英語
英語 フランス
フランス スペイン
スペイン ロシア
ロシア 韓国
韓国 日本
日本






